Diet in IBS Research Study
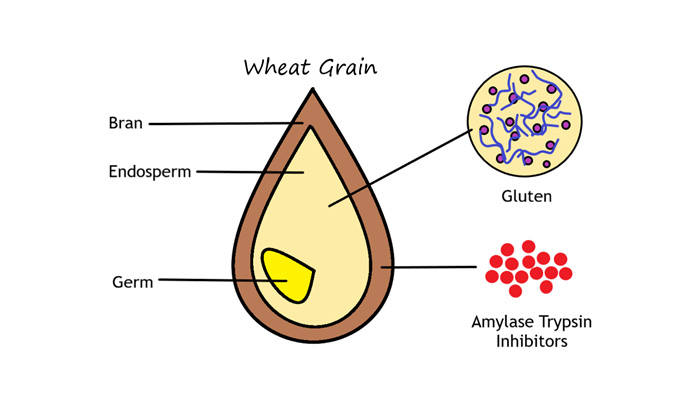
The Diet in IBS Research Study is the first study to investigate the effects of dietary gluten and amylase trypsin inhibitors (ATIs) on irritable bowel syndrome (IBS). The trial is being led by Drs. Premysl Bercik and M. Ines Pinto-Sanchez in the Farncombe Digestive Disease Research Institute at McMaster University.?
What is IBS?
IBS is a common gastrointestinal disease with symptoms including constipation, diarrhea, gas, bloating, and abdominal pain for at least 3 months. IBS is difficult to treat because it is very diverse and it is likely that different subgroups of patients should have personalized treatments to help improve their symptoms. Many IBS patients experience symptoms after they eat certain foods, prompting them to avoid those foods. Some common “trigger foods” include dairy, eggs, beans, fried or fatty foods, alcohol, spicy foods, processed foods, and gluten.
Further information and answers to FAQs can be found here.
Gluten-free diet for IBS
A gluten-free diet is effective at improving symptoms in a proportion of IBS patients. However, it’s currently unknown whether gluten itself or other components of wheat are the cause for IBS symptoms. Amylase trypsin inhibitors (ATIs) are proteins that have been shown to activate the immune system in animal studies. They are found in all of the gluten-containing grains (wheat, rye, & barley), so it may be that ATIs cause or enhance IBS symptoms instead of gluten. ATIs do not have the same unique baking properties of gluten, so if they cause symptoms in patients then it would be possible to develop ATI-free flour for baking. This study will be the first to test whether ATIs or gluten cause symptoms in IBS.
Eligibility Criteria:
- Adults aged 18+
- Diagnosed with IBS by your physician
- Experiences an improvement in symptoms on a gluten-free diet
Study Duration:
6 visits over 7-10 weeks
Samples to be collected:
6 blood samples, 6 urine samples, 6 stool samples, & 4 x-rays
Compensation
We will offer compensation and parking passes will be provided
Contact us to know further information about the study:
Research Coordinator: Caroline Seiler, PhD Candidate ibs@mcmaster.ca
Phone: (905) 580-0325
Sponsor Funding/Funding Disclosures:
This study is supported by the Canadian Digestive Health Foundation and a Society for the Study of Celiac Disease (SSCD) grant for Non-Celiac Gluten/Wheat Sensitivity sponsored by the Nestle Research Center, Nestec SA to Drs. Bercik and Pinto-Sanchez.
Research Team:
Ms. Caroline Seiler, Dr. Premysl Bercik, Dr. Maria Ines Pinto-Sanchez, Dr. Elena Verdu, Dr. Stephen Collins, Dr. Paul Moayyedi, Dr. Andrea Nardelli, Dr. Detlef Schuppan, Dr. Pedro Miranda, Dr. Gaston Rueda, & Ms. Rajka Borojevic
This study was approved by the Hamilton Integrated Research Ethics Board (#4367).
Clinical Studies, Farncombe Family Digestive Health Research Institute, ResearchRelated News
News Listing

McMaster researchers develop new, publicly accessible AI tool to help scientists find new antibiotics
Feature, Research
5 days ago

Brighter World ➚
Analysis: Ontario’s high-stakes bet on iGaming: Province profiting from online betting but at what cost to problem gambling?
Research
January 20, 2025

The power of cinema: Study shows film intervention reduces violence against children
Dept. Psych, Offord Centre for Child Studies, Research
January 17, 2025
